CRMs for medical devices
A collaboration project between JRC and University of Pisa
Critical raw materials in medical devices
Ensuring healthy lives and promote well-being is one of the Sustainable Development Goals under the UN 2030 Agenda for Sustainable Development. Raw Materials (RM) can play an important role in contributing to SDGs, as described in this RMIS section.
In healthcare, medical devices are essential to provide diagnose, treat patients and help them overcome sickness or disease, thus improving their quality of life.
In the EU, Critical Raw Materials (CRMs) are defined as materials with relatively high supply risk and high economic importance for the EU. Moreover, they have very limited or no options for substitution. Some CRMs have peculiar properties like superconductivity, biocompatibility and exceptional resistance and are used high-tech medical devices. For instance, niobium-titanium alloys are employed in magnetic resonance imagers; tantalum is used in prosthetic devices for humans.
The development of next generation medical devices using specific materials is rapidly moving forward and is going to impact the EU citizens' quality of life and access to therapy, as well as healthcare and social costs. At the same time, the production phase of these materials can generate negative impacts from a social and environmental point of view in the countries where they are extracted. Responsible sourcing and security of supply are therefore crucial conditions to ensure that raw materials can positively contribute to SDGs.
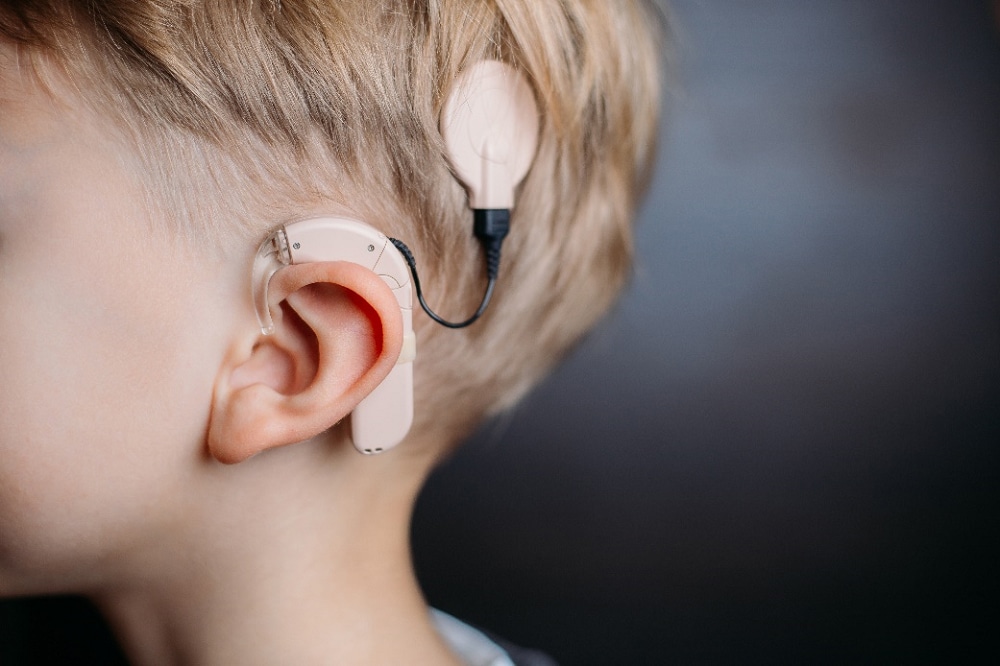
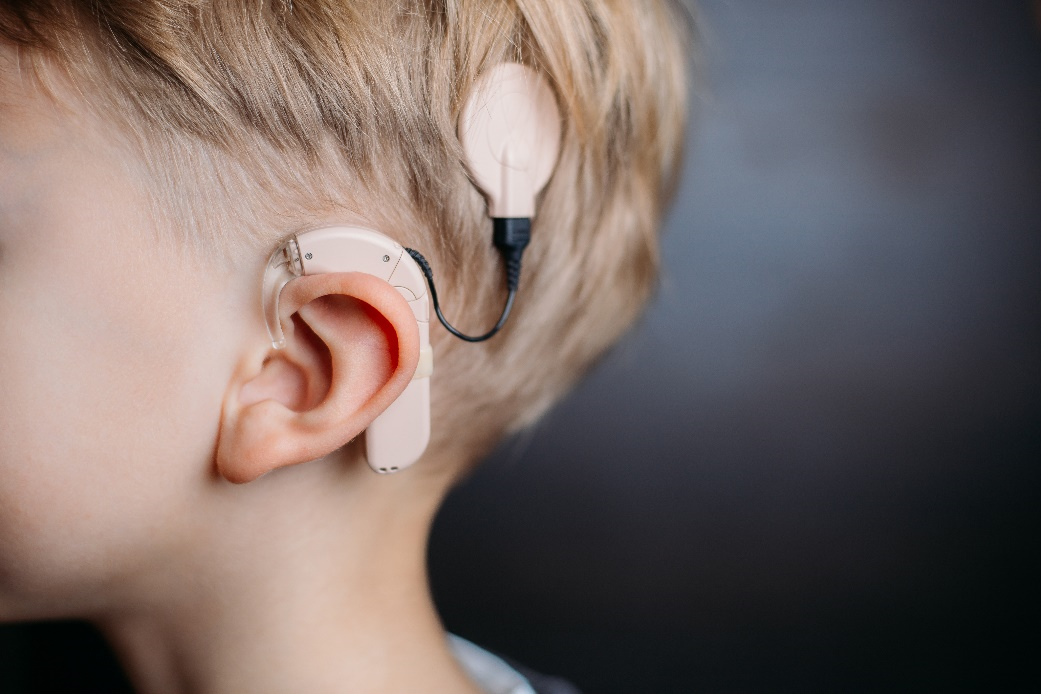
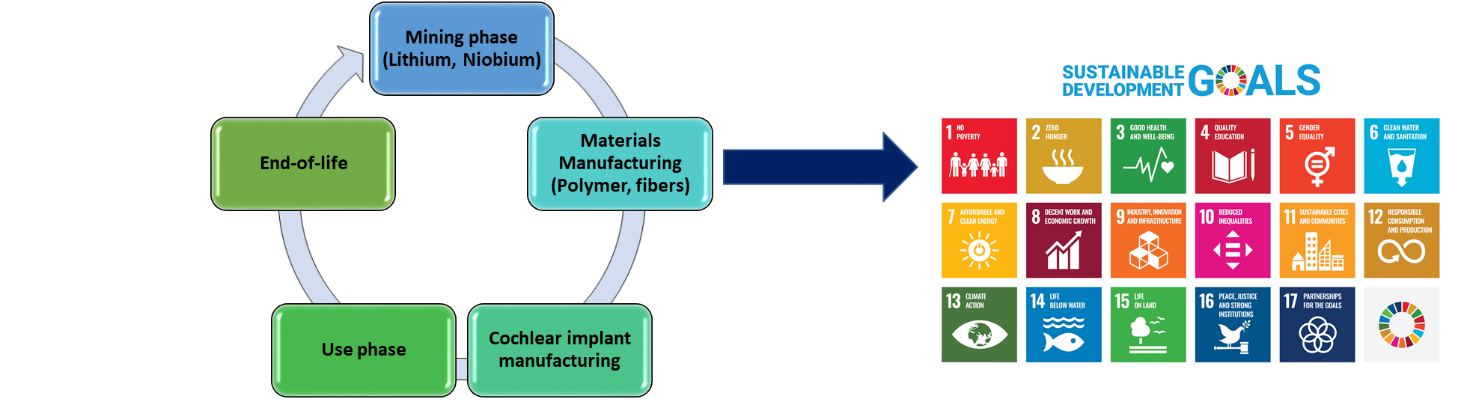
A collaboration project between JRC and Pisa University will investigate the role of lithium-niobate in innovative Cochlear Implants (CIs), with the aim of analyzing the social impacts of this innovative devise along its life cycle, compared to a traditional device (fig. 1).
Lithium Niobate in innovative cochlear implants
Hearing loss is a common problem generally associated with increasing age. Its frequency varies from 0,2% under 5 years of age to over 40% in persons older than 75 years. The incidence of congenital severe to profound hearing loss is about 1-3/1.000 newborns.
A CI is a high-tech multi-component electronic device, with a part permanently implanted in the temporal bone. It can help to provide a sense of sound and support speech to profoundly hearing impaired patients. It is constituted by an external portion, that sits behind the ear and an internal portion surgically placed under the skin.
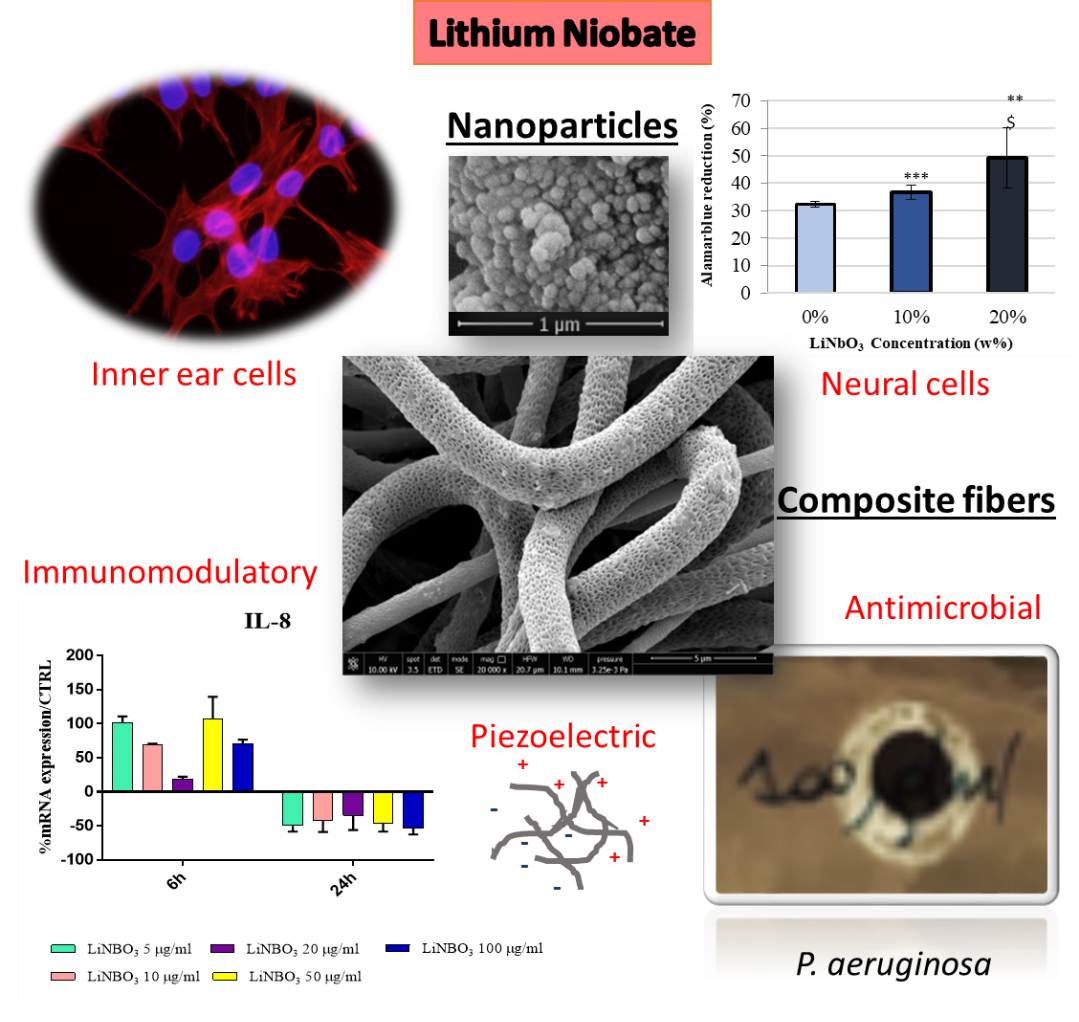
Due to technology inherent limitations the hearing quality is not optimal and several drawbacks still exist. Next generation cochlear implants aim to exploit a different principle of function using piezoelectric materials. Danti et al. (2020) tested lithium niobate nanoparticles as an interface material for these inner ear devices.
Lithium niobate nanoparticles (fig. 2) are a piezoelectric ceramic material considered promising for biomedical implants due to their advantages of being safe for implantation, non-ferromagnetic, and for cochlear implants, efficient in converting vibrations to electric signals. Moreover, lithium niobate nanoparticles are non-toxic and help promote robust immunomodulation and antimicrobial activity against harmful bacteria.
The possible transition from an electronics-based to a materials-based (piezoelectric) CI has been hypothesized in early 2000s. Even though not yet developed at a bed-side stage, piezoelectric CIs aim to change the surgical and clinical management of deafness by providing a self-powered auditory stimulation.
Life-cycle based analysis of social impacts in innovative Lithium Niobate Cochlear Implants (Li-Nb CIs)
Due to the novelty of this technology and the expected increase of smart materials-based devices, sustainability aspects linked to this innovative device have not been studied before. A prospective Life Cycle-based approach can be used to assess the environmental and social performance of an emerging technology to, e.g., inform technology developers of beneficial changes at an early stage of development and push manufacturers toward the most sustainable application of a technology (Caldeira et al. 2018).
Moreover, the UN 2030 Agenda for Sustainable Development offers a unique framework to assess production and consumption system in a comprehensive manner, considering environmental, economic and social impacts simultaneously.
The collaboration between JRC and Pisa University will allow combining the expertise of Pisa University concerning Health Technology Assessment of CIs and piezoelectric CIs with the experience in sustainability assessment of raw materials developed at the JRC. The project will focus on the social impacts of new CIs (niobium based) from a life cycle perspective, thus analysing:
- From the consumption perspective, how this technology could impact the society in terms of improved quality of life and access to medical treatment, and its benefits and risks compared to the traditional implants.
- From the production perspective, what are the main positive and negative impacts along the supply chain and considering in particular the extraction and processing of the two critical raw materials contained in the implant: niobium and lithium.
This analysis will use data published in the literature and derived from the clinical research and data from medical devices producers on the material components. The analysis could also help to build on social models in the area of new medical devices.
The study will analyse, in each life cycle stage, how this technology could positively or negatively contribute to the relevant SDGs (fig. 3). We will then map and identify social impacts and points of improvement along the supply chain, using indicators recommended by the UNEP Guidelines for Social Life Cycle Assessment of Products and Organizations (2020).
Preliminary results
As a first step of the analysis, we identified the relevant SDGs impacted along the whole life cycle of the Li-Nb CIs and considering the stakeholders categories recommended by the Social Life Cycle Assessment Guidelines (UNEP 2020):
- Workers
- Local communities
- Value chain actors
- Consumers
- Society
- Children.
We focused on potential positive and negative social impacts and applied the framework proposed by UNEP (2020), linking SDGs to S-LCA impact subcategories. We then assessed which goals are relevant for the various stakeholders and life cycle stages (Fig. 4).
For instance, considering the extraction on materials used in the Li-Nb cochlear implants (i.e. mining phase) and the stakeholder “Workers”, the following Goals and S-LCA impact subcategories can be considered as relevant for the impact assessment:
- Goal 1 – No poverty (impact subcategories: Fair salary; Poverty alleviation)
- Goal 3 – Good health and well-being (impact subcategory: Workers health and safety)
- Goal 5 – Gender equality (impact subcategories: Equal Opportunity / Discrimination; Sexual harassment)
- Goal 8 – Decent work and economic growth (impact subcategories: Freedom of association; Child labour; Forced labour; Working hours; Social benefits/security; Fair salary; Employment relationships).
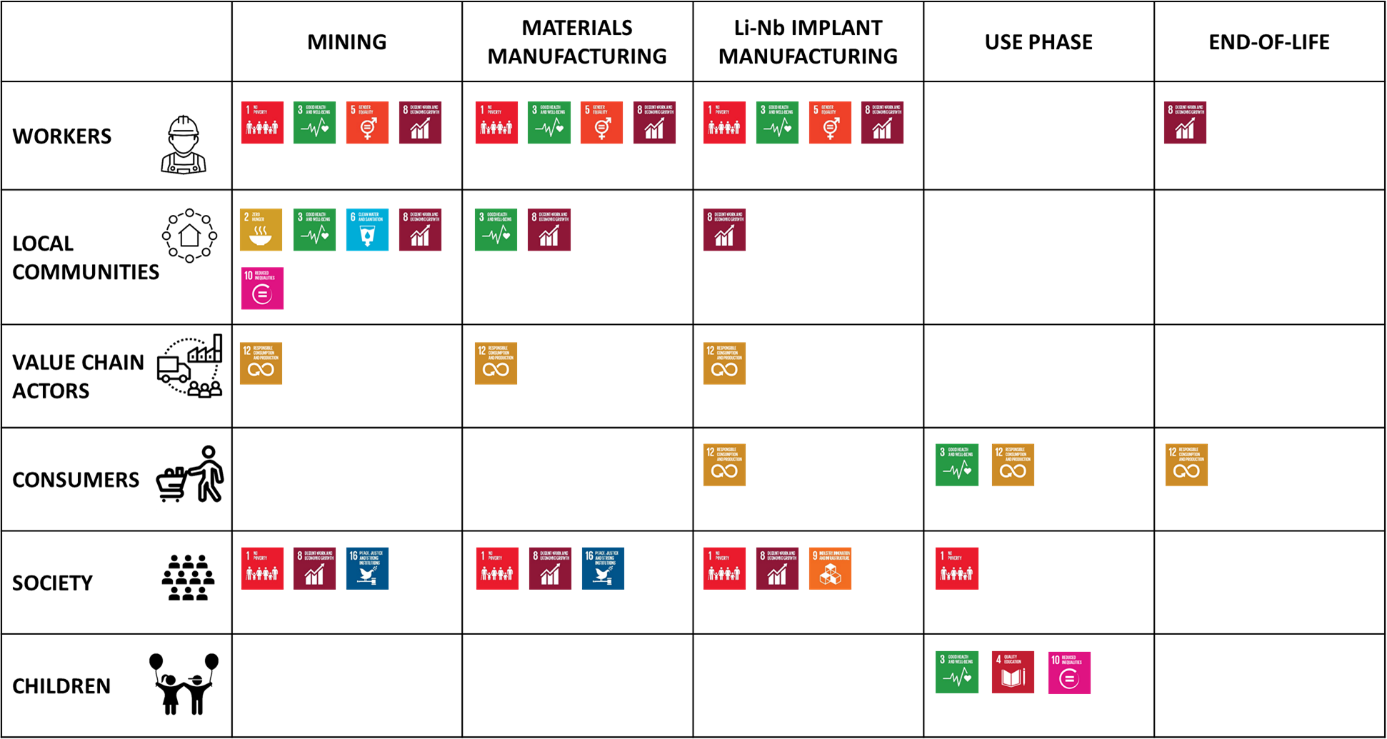
Focusing on one Goal, specifically Goal 3 on Good health and well-being, fig. 5 shows how the assessment is performed: starting from relevant stakeholders categories (icons) and taking into account related impact subcategories, appropriate indicators and metrics can be developed and the corresponding risk level can be assessed.
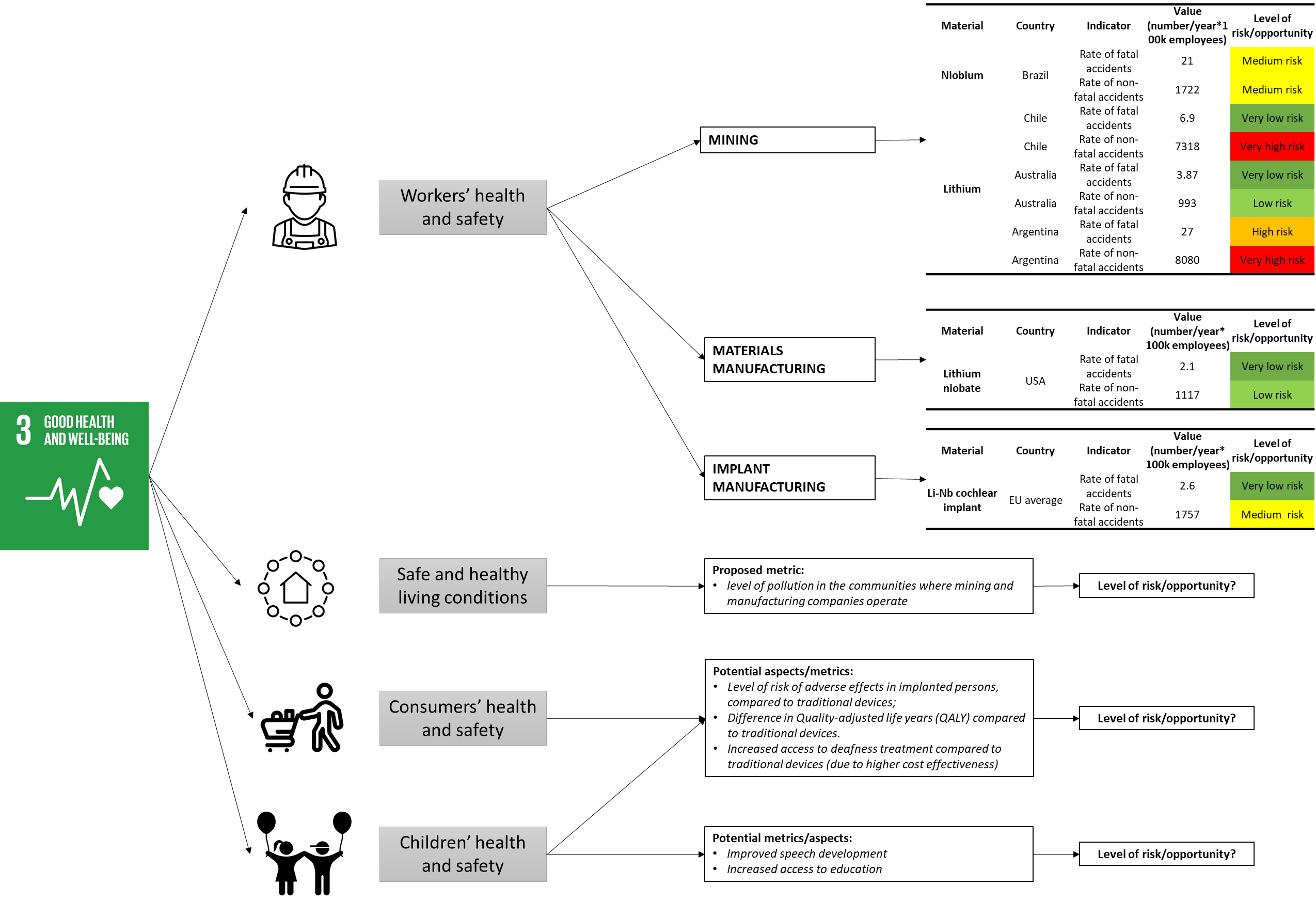
In the case workers’ health and safety, the main producing countries are identified in each life cycle stage, based on data published in EC (2020). The indicators “Rate of fatal accident at work” and “Rate of non-fatal accident at work” are used to assess the impact category workers’ health and safety. Sector-specific values for accident at work are provided by the PSILCA database, based on latest ILO estimates. The risk assessment is based on the following scales, provided by the PSILCA v.2 database documentation.
Table 1 Risk assessment reference scale for fatal accidents (Source PSILCA v.2 database documentation)
|
Fatal accidents Indicator value y (# of accidents/ year 100k employees) |
Risk level |
| 0 = y < 7.5 | Very low risk |
| 7.5 = y < 15 | Low risk |
| 15 = y < 25 | Medium risk |
| 25 = y < 40 | High risk |
| 40 = y | Very high risk |
Table 2 Table 1 Risk assessment reference scale for non-fatal accidents (Source PSILCA v.2 database documentation)
|
Non-fatal accidents Indicator value y (# of accidents/ year 100k employees) |
Risk level |
| 0 = y < 750 | Very low risk |
| 750 = y < 1500 | Low risk |
| 1500 = y < 2250 | Medium risk |
| 2250 = y < 3000 | High risk |
| 2250 = y < 3000 | Very high risk |
In the case of other stakeholder categories, potential metrics and indicators are proposed for the risk and opportunities assessment. In the case of consumers and children, the following aspects are identified as relevant for the assessment:
- Level of risk of adverse effects in implanted persons, compared to traditional devices
- Difference in Quality-Adjusted Life Years (QALY) compared to traditional devices
- Increased access to deafness treatment compared to traditional devices (due to higher cost effectiveness)
- Improved speech development in children
- Increased access to education for children.
Given that the Li-Nb CI is a new technology in its testing phase, there are no primary data available to perform the assessment for the use phase. The level of risk/opportunity of the above mentioned aspects, could, however, be estimated based on the results of the clinical trials and also comparing the performances of the traditional electronic devices.
Research team and contacts
Serena Danti, University of Pisa, Department of Civil and Industrial Engineering, serena.danti@unipi.it
Bahareh Azimi, University of Pisa, Department of Civil and Industrial Engineering, b.azimi@ing.unipi.it
Stefano Berrettini, University of Pisa, Department of Surgical, Medical, Molecular Pathology and Emergency Medicine, s.berrettini@med.unipi.it
Lucia Mancini and Simone Manfredi, Joint Research Centre, Land Resources Unit, simone.manfredi@ec.europa.eu
